A simple two-step reaction

A turns into B, with rate constant k1, which turns into C, with rate constant k2.

A turns into B, with rate constant k1, which turns into C, with rate constant k2.
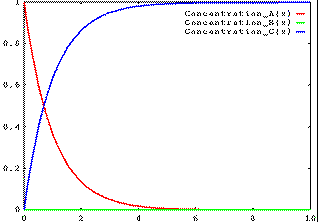
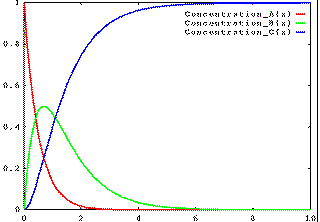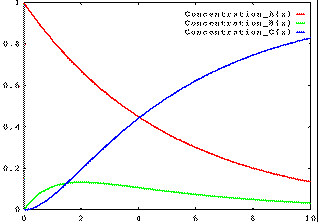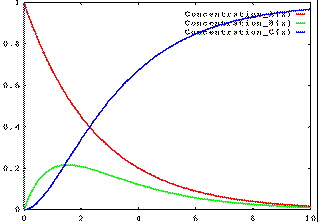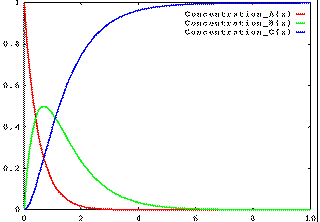
If k1 is slow and k2 is fast, then the concentration of B is always low, as is illustrated in this figure.
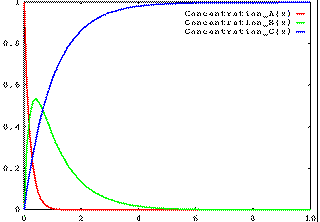
Alternatively, if k1 is fast and k2 is slow, then the concentration of B will build up, then fall away:
To experiment with different values of k1 and k2, type:
demo1 k1 k2
demo2
The demo version is rather smoother than this image:
demo3d