| Ce | Cerium |
Atomic number : 58
Relative atomic mass (12C = 12.0000) : 140.115 |
|||||||
| P H Y S I C A L D A T A | |
|
Group 3 lanthanide Density / kg m-3 : 8240 (  ); 6749 ( ); 6749 (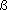 ); 6773 ( ); 6773 ( ); 6700 ( ); 6700 ( ) [298 K] ) [298 K] |
Melting point / K : 1072 Boiling point / K : 3699 |
| D I S C O V E R Y | |
|
Cerium was discovered in 1803 by J.J. Berzelius and W. Hisinger at Vestmanland, Sweden. |
|
| G E O L O G I C A L D A T A | |
|
Abundances Sun (relative to H = 1 × 1012) : 35.5 Earth's crust / p.p.m. : 68 Residence time / years : 100 Classification : scavenged Oxidation state : III |
Seawater / p.p.m. : Atlantic surface : 9.0 × 10-6 Atlantic deep : 2.6 × 10-6 Pacific surface : 1.5 × 10-6 Pacific deep : 0.5 × 10-6 |
| B I O L O G I C A L D A T A | |
|
Level in humans Blood / mg dm-3 : < 0.002 Bone / p.p.m. : 2.7 Liver / p.p.m. : 0.29 Muscle / p.p.m. : n.a. |
Daily dietary intake : n.a., but very low Total mass of element in average (70 kg) person : 40 mg |
