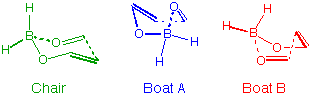

The Idea
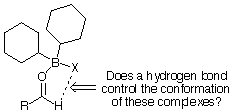
Figure 2 Hydrogen bond from formyl hydrogen
C-H hydrogen bonds have been the subject of a number of reviews [7]. Recent publications by Corey [8] suggest that an interaction between the electronegative
group on the boron and the hydrogen of the aldehyde may be important in systems such as enol-borinates coordinated to aldehydes.
These papers have lead us to reconsider our earlier explanation for the geometry of such Lewis-acid
complexes [9].
We have quantified this hydrogen-bonding interaction for H2BF complexes
[10], and these results have implications for the properties of
the aldol reactions on enol-borinates.
The interaction is not included in the transition state force field for the
aldol reaction, but it should be present. The addition of such an interaction would have little
effect on the Chair and Boat-A transition states (Figure 3), but would have a stabilising effect on Boat-B,
because this is the only geometry for which the hydrogen of the aldehyde is close to the
oxygen of the enol-borinate. This, therefore, represents a physically significant way of stabilising
the Boat-B transition state without effecting the other transition states. In addition, this should
have no effect on allylborination transition states, so this model [3] does not need to be revised in the
light of this new idea.
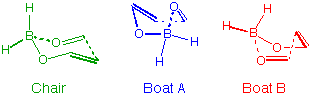
Figure 3 The three transition structures for the boron-mediated aldol reaction
Methodology
The force-field for the boron-mediated aldol transition state is distributed with
MacroModel [11], as a modification to the MM2* force field [12]. For this study, we made a minor alteration to the
standard force field:
the B-O bond connecting the boron to the aldehyde was made a single bond, not a zero order bond.
This makes conformation searching much easier, because the transition state
can now be considered as a ring, and not as two separate molecules. This also
makes comparisons (especially superpositions) of the results of the searches easier.
It has no effect on the geometries and energies of the transition structures that are generated by the force field.
In order to allow for the new interaction between the formyl C-H of the aldehyde and
the oxygen of the enol-borinate, we introduced an harmonic constraint between these
two atoms. We chose a separation of 2.4 Å as the bottom of the harmonic potential [9]. A cut-off of 2.9 Å was manually introduced. This means that the new interaction has no effect on chair and boat A transition structures. A fairly weak force constant of 25 kJ/mol was chosen.