The new method was applied to a variety of aldol reactions, using experimental data from Brown (Figure 5) [13]. For each system, a conformation search was carried out with the constraint in place.
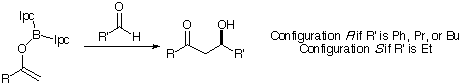
Figure 5: RCOMe reacts with R'CHO
| RCOMe | R'CHO | Experimental | Monte Carlo | Constrained Monte Carlo | Filtered |
|---|---|---|---|---|---|
| Me | Et | 62 % S | 33 % R | 6 % S | 15 % S |
| Me | i-Pr | 61 % R | 45 % S | 0.4 % R | 10 % R |
| Me | t-Bu | 83 % R | 7 % R | 15 % R | 19 % R |
| Me | Ph | 57 % R | 35 % S | 13 % S | 29 % S |
| Et | Et | 61 % S | 16 % R | 10 % S | 16 % S |
| Et | i-Pr | 48 % R | 13 % R | 21 % R | |
| Et | t-Bu | 81 % R | 18 % S | 16 % S | |
| Et | Ph | 39 % R | 33 % R | 65 % S | 13 % S |
| Ph | Et | 65 % S | 37 % R | 13 % S | |
| Ph | i-Pr | 74 % R | 60 % S | 16 % R | |
| Ph | t-Bu | 89 % R | 19 % S | 21 % R | 63 % R |
| Ph | Ph | 58 % R | 45 % S | 65 % S | 41 % S |
| t-Bu | Et | 17 % S | 3 % R | 4 % R | |
| t-Bu | i-Pr | 13 & R | 15 % S | 16 % S | |
| t-Bu | t-Bu | 26 % R | 54 % S | 53 % S | |
| t-Bu | Ph | 4 % R | 12 % S | 28 % S | 28 % S |
It is clear from Table 2 that the improved force field does not completely account for the selectivity of this reaction. In particular, it appears to be confused by phenyl groups and tertiary-butyl groups. However, It represents a considerable improvement on the original force field.
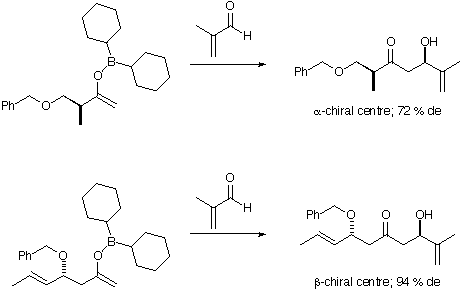
Figure 6: Aldol reactions of chiral ketones
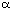 -chiral centre. For the
-chiral centre. For the 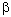 centre, however, the incorrect sense of induction is calculated. The effects of a
centre, however, the incorrect sense of induction is calculated. The effects of a
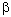 -chiral
centre must depend on long range interactions to a greater extent that the effect of an
-chiral
centre must depend on long range interactions to a greater extent that the effect of an
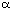 -chiral centre, and so the force field may be expected to be less accurate. Further investigation is required to optimise the improvements to the force field.
-chiral centre, and so the force field may be expected to be less accurate. Further investigation is required to optimise the improvements to the force field.