| S | Sulfur |
Atomic number : 16
Relative atomic mass (12C = 12.0000) : 32.066 |
|||||||
| P H Y S I C A L D A T A | |
|
Group 16 non-metal Density / kg m-3 : 2070 (  ), 1957 ( ), 1957 (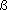 ) [293 K]; 1819 [liq., 393 K] ) [293 K]; 1819 [liq., 393 K] |
Melting point / K : 386.0 ( ); 392.2 ( ); 392.2 (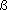 ); 380.0 ( ); 380.0 ( ) ) Boiling point / K : 717.824 |
| D I S C O V E R Y | |
|
Known to ancient civilizations. |
|
| G E O L O G I C A L D A T A | |
|
Abundances Sun (relative to H = 1 × 1012) : 1.6 × 107 Earth's crust / p.p.m. : 260 Residence time / years : 8 × 106 Classification : accumulating Oxidation state : VI |
Seawater / p.p.m. : 870 |
| B I O L O G I C A L D A T A | |
|
Level in humans Blood / mg dm-3 : 1800 Bone / p.p.m. : 500 - 2400 Liver / p.p.m. : 7000 - 12 000 Muscle / p.p.m. : 5000 - 11 000 |
Daily dietary intake : 850 - 930 mg Total mass of element in average (70 kg) person : 140 g |
