Alkane Global Minima
What is the global minimum conformation of an unbranched alkane, length N ?
Jonathan M Goodman
Department of Chemistry, Lensfield Road, Cambridge
Studies on short alkanes show that the global minima for
chains of less than eighteen carbon atoms are extended conformations. Longer chains
prefer hairpin-like conformations. Longer chains are likely to have more complicated
global minima structures.
Questions
- What is the shortest chain whose global minimum is neither extended nor a hairpin? (Prize A)
- What chain length has the highest global minimum energy ? (Prize A)
- What is the shortest chain with a negative global minimum energy? (Prize A)
- Can you find a lower energy structure for: (Prize B)
- What is the global minimum energy structure for CnH2n+2 ? (Prize C)
Energies must be calculated using the MM2* force field, as implemented in
MacroModel version 5.5.
All energies are in kJ/mol
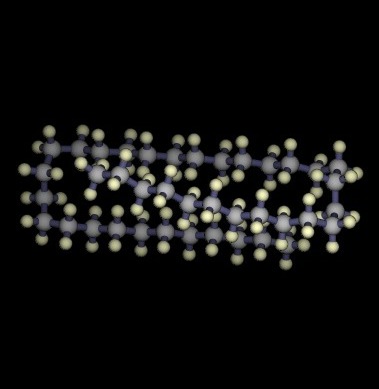
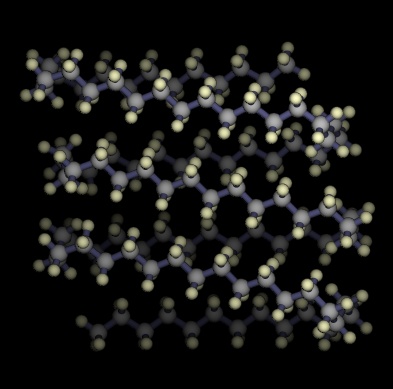
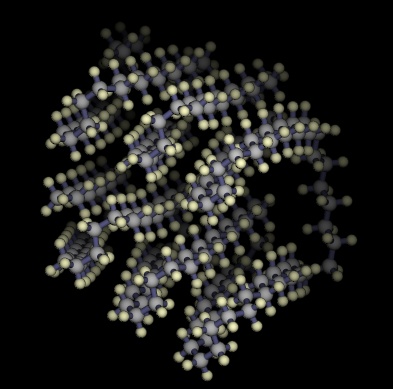
Lowest energy structures known for unbranched alkanes
 |
 |
 |
C39H80
58.98 kJ / mol |
C100H202
36.06 kJ / mol |
C200H402
-59.78 kJ / mol |
Prizes
All prizewinners will receive the fame and fortune which results from
having their achievements listed on these web pages! In addition:
- Prize A: The sender of the best answer to each question received by December 25th, 1997, will win a glass of lemonade and a packet of crisps, provided at a time convenient to both the prize winner and the organiser, at a pub within 200 yards of the University Chemistry Laboratory, Cambridge.
- Prize B: The first ten distinct answers received (duplicates will be discarded, even if submitted by different people) will each win a cup of tea or coffee, provided at a time convenient to both the prize winner and the organiser, in the tea room of the University Chemistry Laboratory, Cambridge.
- Prize C: The winner may offer a new prize to anyone finding a lower energy structure!
Please note that the organisers are not responsible for any costs incurred in collecting the prizes
Rules
- Energies must be calculated using the MM2* force field, as implemented on
MacroModel version 5.5
- The lowest energy conformations known are listed on the web page:
http://www-jmg.ch.cam.ac.uk/stuff/alkanes/news.html
- If you find a lower energy structure for an unbranched alkane than any of those listed here, send the geometry and energy to jmg11@cam.ac.uk.
The work "ALKANES" (capital letters) should be the first word of the subject line.
- If you do not have access to
MacroModel
version 5.5, you could use another implementation of MM2 or a related force field. These
are likely to give different absolute values for the energy, which cannot be directly compared
with those listed on these web pages. In order to enter the competition:
- Use whatever molecular mechanics program you can to calculate the energies of
some of the alkanes listed here, in order to calibrate your force field.
- If you find a structure with a lower energy than one of these, using your force field,
send the structure (as a MacroModel, PDB or MOL format file) to
jmg11@cam.ac.uk, giving details of the
program that you used to calculate the energy, and the energy you obtained. Promising
structures will be reminimised using MacroModel, and recorded here, if they are lower
in energy according to MacroModel.
- The algorithm implemented in MacroModel 5.5 to calculate the energy is
described in Allinger's paper: J. Am. Chem. Soc. 1977, 99, 8127.
Details of the components of the energy for butane are available.